叔丁哌苯
| 此條目可参照德語維基百科相應條目来扩充。 (2024年1月22日) |
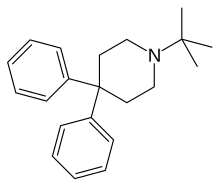
叔丁哌苯(英語:Budipine,也译为布地平)商品名Parkinsan,分子式C21H27N,是一种帕金森氏症治疗药物[2][3],其作用机制尚未解明[4]。
 | |
| 臨床資料 | |
|---|---|
| AHFS/Drugs.com | 国际药品名称 |
| ATC碼 | |
| 识别信息 | |
| CAS号 | 57982-78-2 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.055.494 |
| 化学信息 | |
| 化学式 | C21H27N |
| 摩尔质量 | 293.45 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |

参考文献
编辑- ^ Sweetman SC (编). Martindale: The Complete Drug Reference. 35th. London: Pharmaceutical Press. 2007. ISBN 978-0-85369-687-2.
- ^ Przuntek H, Müller T. Clinical efficacy of budipine in Parkinson's disease. Journal of Neural Transmission. Supplementum. 1999, 56: 75–82. ISBN 978-3-211-83275-2. PMID 10370903. doi:10.1007/978-3-7091-6360-3_3.
- ^ Budipine. AdisInsight. Springer Nature Switzerland AG. [2023-08-21]. (原始内容存档于2023-05-07).
- ^ Reichmann H. Budipine in Parkinson's tremor. Journal of the Neurological Sciences. October 2006, 248 (1–2): 53–55. PMID 16784759. S2CID 21540225. doi:10.1016/j.jns.2006.05.039.
- ^ Schaefer H, Hackmack G, Eistetter K, Krüger U, Menge HG, Klosa J. [Synthesis, physical-chemical properties and pharmacologically-oriented screening studies on budipine and related 4,4-diphenylpiperidines]. Arzneimittel-Forschung. 1984, 34 (3): 233–240. PMID 6539602 (德语).