User:冰霜葵/沙盒叄
| 乙酸汞 | |||
|---|---|---|---|
| |||
| |||
| 英文名 | Mercury(II) acetate | ||
| 别名 | 乙酸高汞 乙酸汞(II)盐 醋酸汞 二乙酰基氧基汞 双(乙酰氧基)汞 乙酸汞(2+)盐 二乙酸汞 mercuric acetate mercuriacetate | ||
| 识别 | |||
| CAS号 | 1600-27-7 | ||
| ChemSpider | 14599 | ||
| SMILES |
| ||
| InChI |
| ||
| InChIKey | BRMYZIKAHFEUFJ-NUQVWONBAS | ||
| 性质 | |||
| 化学式 | C4H6O4Hg | ||
| 摩尔质量 | 318.70 g·mol⁻¹ | ||
| 外观 | 白色晶體或粉末[1] | ||
| 密度 | 3.27 g/cm³,固體 | ||
| 熔点 | 179℃(分解) | ||
| 溶解性(水) | 25 g/100 mL(10℃) | ||
| 溶解性 | 溶於乙醇 | ||
| 危险性 | |||
| 警示术语 | R:Template:R-p | ||
| 安全术语 | S:Template:S-p | ||
| NFPA 704 | |||
| 致死量或浓度: | |||
LD50(中位剂量)
|
76 mg/kg(大鼠經口) | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
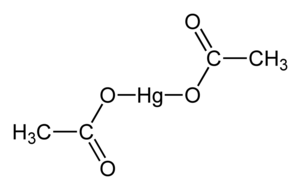
乙酸汞,化學式 Hg(O2CCH3)2 。通常縮寫成 Hg(OAc)2 ,this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors.
性質 编辑
Arenes undergo "mercuration" upon treatment with Hg(OAc)2. The one acetate group that remains on mercury can be displaced by chloride:[2]
- C6H5OH + Hg(OAc)2 → C6H4(OH)-2-HgOAc + HOAc
- C6H4(OH)-2-HgOAc + NaCl → C6H4(OH)-2-HgCl + NaOAc
The Hg2+ center binds to alkenes, inducing the addition of hydroxide and alkoxide. For example, treatment of methylacrylate with mercuric acetate in methanol gives an α-mercuri ester:[3]
- Hg(OAc)2 + CH2=CHCO2CH3 + CH3OH → CH3OCH2CH(HgOAc)CO2CH3 + HOAc
Mercury(II) has a high affinity for sulfur ligands. Hg(OAc)2 can be used as a reagent to remove the acetamidomethyl protecting group, which is used to "protect" thiol groups in organic synthesis. Similarly Hg(OAc)2 is a standard reagent to convert thiocarbonate esters into dithiocarbonates:
- (RS)2C=S + H2O + Hg(OAc)2 → (RS)2C=O + HgS + 2 HOAc
結構 编辑
Mercury(II) acetate is a crystalline solid consisting of isolated Hg(OAc)2 molecules with Hg-O distances of 2.07 Å. Three long, weak intermolecular Hg···O bonds of about 2.75 Å are also present, resulting in a slightly distorted square pyramidal coordination geometry at Hg.[4]
參考文獻 编辑
- ^ (简体中文)化工词典 乙酸汞
- ^ Whitmore, F. C.; Hanson, E. R. "o-Chloromercuriphenol" Organic Syntheses, Collected Volume 1, p.161 (1941).http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV1P0161
- ^ Carter, H. E.; West, H. D. “dl-Serine” Organic Syntheses, Collected Volume 3, p.774 (1955). http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV3P0774
- ^ R. Allmann. Z. Kristallogr., Kristallgeom., Kristallphys., Kristallchem. 1973, 138: 366–373. 缺少或
|title=为空 (帮助)


